There are 4 phases in clinical trials:




Phase 1 A small number of healthy volunteers are recruited. Absorption, distribution, metabolism and excretion of investigational drug or treatment in the human body are examined through observation of clinical signs and examination, in order to assess their safety, determine the best dose and evaluate side effects. This phase must be conducted in hospitals.
Phase 2 Small-scale recruitment of volunteers with particular illnesses as study subjects. Generally using a control-group design, compares an investigational drug or treatment in various dose forms with an existing treatment or placebo, and further determines the efficacy and safety of investigational drug. This phase can be conducted at clinical trial centers.
Phase 3 Expanded recruitment of hundreds to thousands of patients. Patient will be randomly assigned to experimental or control groups in a double-blind design (i.e., healthcare professionals and patients will not be aware of group assignment). Comparisons are made with existing standard treatments to further test the efficacy of investigational drug, and monitor side effects to confirm whether the investigational drug or treatment is better or not worse than existing treatment options. This is also the final stage before the experimental drug or treatment is officially introduced to the market. The safety of the drug is usually known to the research team at the time of the study. These studies can be done at clinical trial centers.
Phase 4
Large-scale safety tracking studies are conducted after a drug has been officially
approved for use to assess the long-term effects of the treatment.
Most of the studies conducted by the Hong Kong Clinical Research Center are Phase II and
Phase III studies.
Clinical study participants can play an active role in research, gain access to new treatments
and contribute to the well-being of patients by facilitating medical research, in order to
accelerate the process of bringing new drugs or treatments to the community.
Most of the studies conducted by the Hong Kong Clinical Research Center are Phase II and Phase III studies. Clinical study participants can play an active role in research, gain access to new treatments and contribute to the well-being of patients by facilitating medical research, in order to accelerate the process of bringing new drugs or treatments to the community.
Importance of participating in clinical research to individuals and the community
- 1
The latest treatments: access to the latest treatments that may be more effective, safer, or more convenient than existing treatments
- 2
Professional medical care with frequent check-ups: professional medical care at no cost to you, with researchers (including doctors and nurses) closely monitoring your health and providing necessary treatment and care.
- 3
Compensation: Most clinical research studies provide subsi, such as transportation costs.
- 4
Self and community contribution: Participating in a clinical study helps researchers better understand the disease, which can lead to better treatments and benefit more patients.
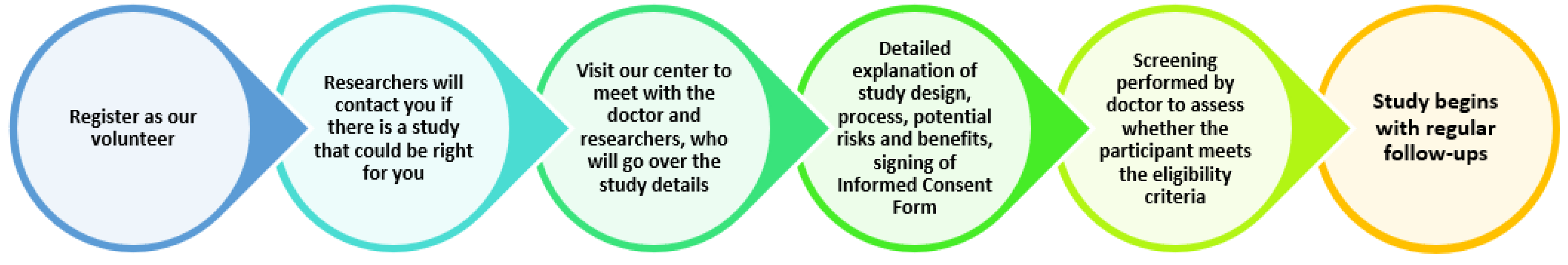
Process of participating in a clinical trial:
FAQ
How do researchers ensure the safety of participants?
Who can participate in a clinical trial?
Do I have the right to withdraw from the study once I have participated in it?
What should I consider before participating in a clinical study?
Click the link or scan QR code below to register as a clinical trial volunteer now!
Confidentiality of personal data Limited to the organizations mentioned in the consent form (e.g. our center, sponsors, research-related institutions) which have limited rights to obtain your personal data to ensure that personal data is kept confidential and protected. .